Nkọwa nke In Vitro Diagnostic
In Vitro Diagnosis (IVD) na-ezo aka na usoro nyocha nke na-enweta ozi nyocha ụlọ ọgwụ site n'ịchịkọta na nyochaa ihe ndị dị ndụ, dị ka ọbara, mmiri mmiri, ma ọ bụ anụ ahụ, iji chọpụta, gwọọ, ma ọ bụ gbochie ọnọdụ ahụike.Nchọpụta nke in vitro bụ isi iyi dị mkpa nke nyocha ụlọ ọgwụ, nke nwere ike ịnye ntụnye ntụaka dị mkpa maka atụmatụ ọgwụgwọ ndị dọkịta.IVD bụ akụkụ dị mkpa nke usoro ahụike iji hụ na ahụike mmadụ.
Nkeji Market IVD
Dabere na nhazi nke ụkpụrụ ule, enwere ike kewaa ngalaba ahịa IVD n'ime Microbiology, Clinical Chemistry, Hematology, Coagulation, Immunoassay, Molecular Diagnostics, POCT, wdg Dabere na nhazi nke ngwaahịa nnwale ahụ, enwere ike kewaa ahịa IVD n'ime ya. reagents, ngwa na ọrụ.
Evolution nke IVD
Nkeji 1:
Ihe mepụtara microscope mere ka e nwee ụfọdụ ụzọ nyocha ọdịnala.
Nkeji 2:
Mmepe nke ọgwụ ọgbara ọhụrụ na nchọpụta nke mmeghachi omume enzyme-catalyzed na mmeghachi omume antigen-antibody tọrọ ntọala maka biochemical na immunodiagnosis, ya mere nchọpụta in vitro na-ebili ma jiri nwayọọ nwayọọ gbasaa n'oge a.
Nkeji 3:
Ngwa nke nhazi helix abụọ nke DNA, teknụzụ mgbochi monoclonal na teknụzụ macromolecular na-akwalite mmepe nke nyocha mkpụrụ ndụ na ụlọ ọrụ nyocha vitro.
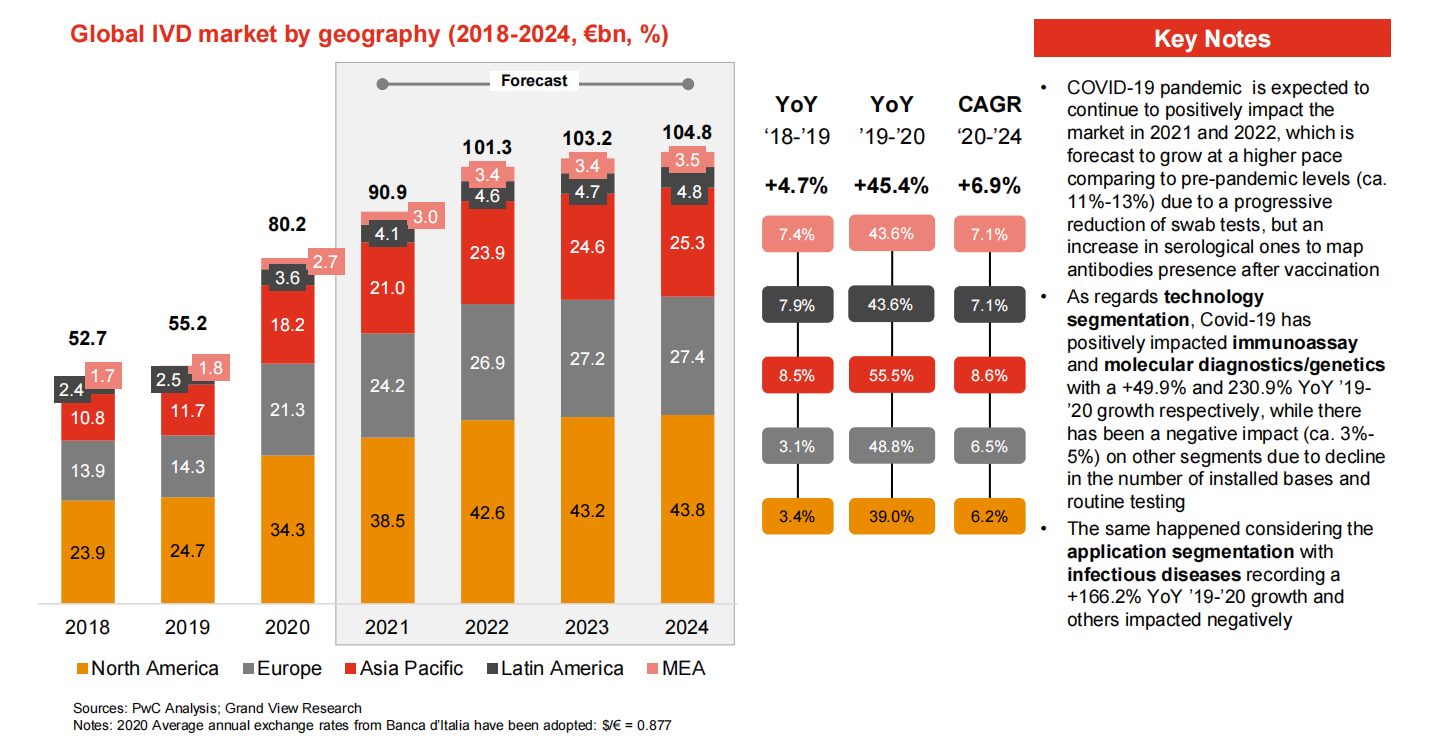
Ahịa IVD zuru ụwa ọnụ
Ihe karịrị 70% nke ahịa IVD zuru ụwa ọnụ bụ ndị Europe, North America na Japan nwere.Ndị isi mba ụwa anọ bụ Roche (Switzerland), Abbott (US), Thermo (US) na Siemens (Germany).Companieslọ ọrụ anọ a nwere mkpokọta ahịa ahịa zuru ụwa ọnụ nke ihe dịka 51% na 2017.




.png)








 Kaadị azụmahịa
Kaadị azụmahịa WeChat ndị China
WeChat ndị China WeChat Bekee
WeChat Bekee